


















In your equation, you've written the conjugate base of hydroxylamine. To make a conjugate acid, add an H+ ion, not take one away (and also make an OH- ion). The equilibrium sign tells you that it is a weak base. 1 0. Anonymous. 4 years ago. Honh2. Source(s): https://owly.im/a7WvA. 0 2. ellen . 6 years ago. sophisticated subject. try searching into google or bing. just that can help! 0 6. Still Answer to 6. Identify the conjugate base of HSO4- A) OH- B) H2SO4 C) H20 D) H2SO3 E) SO42- 7. A 0.14 M HNO2 solution is 5.7% ioniz... What is the conjugate base of HSO4-? SO42-What is the conjugate base of HCO3-? CO32-What is the conjugate acid of CO32-? HCO3-What is the conjugate acid of HCO3-? H2CO3. What is the conjugate acid of H20? H3O+ Double headed arrows mean _____. Weak acids/bases. What is it called when water reacts with itself? Autoionization . Majority of weak acids are in the form of what? Reactants. ALL HSO4- + H + Now, HSO4-is a base since it has the ability to accept a proton but it is a conjugate base to H 2 SO 4 since it is formed by the H2SO4 after donating a proton. The molecule can only resonate through 3 of its Oxygens while the last one holds the hydrogen. Write The Conjugate Acids Of The Following: CH3OH: HSO4- 2. Write The Conjugate Base Of The Following: A) H3PO4 B) CH3NH3+ 3. What Is The Useful Buffering Range For A Buffer Prepared With Formic Acid (Ka = 1.78 X 10-4) 4. Calculate The PH Of A Buffer Solution That Is 0.080 M Sodium Benzoate And 0.020 M Benzoic Acid? The PKa Of Benzoic Acid Is 4.20. In the reaction HCl + NH₃ --> NH₄⁺ + Cl⁻ which is the conjugate acid and which is the conjugate base? Arrhenius acid, Bronsted-Lowry acid. What types of acids is HCl? Bronsted-Lowry base, not an Arrhenius base (because it has no OH in the formula) What types of bases is NH₃? OH⁻, OH⁻ What are the products of the following acid base reaction? O²⁻+H₂O--> HSO₃⁻, HPO₄² B. Loss of a proton from an acid forms its conjugate base. C. Gain of a proton by an acid forms its conjugate base. D. Brønsted-Lowry acid-base reactions always result in the transfer of a proton from a base to an acid. Bloom's Level: 3. Apply Difficulty: Easy Gradable: automatic Section: 02.01 Subtopic: Acid/Base definitions What is the conjugate base of HSO4- and HC2H3O2? What is the conjugate acid of CN- and CO3(2-) Source(s): conjuguate base hso4: https://tr.im/gwKhm. 0 0. Sherry. Lv 4. 5 years ago. complex aspect. seek using yahoo or google. this might help! 0 1. How do you think about the answers? You can sign in to vote the answer. Sign in . greenwhitecollege. Lv 4. 1 decade ago. In HSO4 it is SO4 because it HSO4- is a base since it has the ability to accept a proton but it is a conjugate base to H2SO4 since it is formed by the H2SO4 after donating a proton. The conjugate base of HSO4- is SO4 (2-), if that’s the question that you are asking. 26.8K views View 13 Upvoters Give the conjugate acid and the conjugate base for HSO4-. Answer: conjugate acid: H2SO4. conjugate base: SO42-19 Explain why AlCl3 is a Lewis acid. A Lewis acid is an electron pair acceptor. Aluminum in AlCl3 has an empty p orbital that can accommodate the pair of electrons provided by a Lewis base. 20 E) V. 21 Identify the most acidic carboxylic acid. A) ICH2COOH. B) BrCH2COOH. C) CH3COOH. D
[index] [8864] [9842] [1230] [6277] [2016] [9779] [6071] [7722] [8133] [9603]
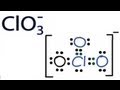
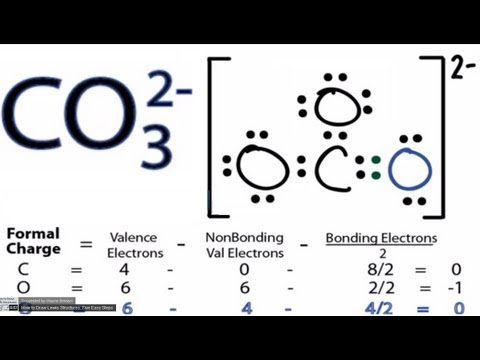
In this video we will look at the equation for HCl + H2O and write the products. When we add HCl to H2O the HCl will dissociate and break into H+ and Cl-. ... This chemistry video tutorial shows you how to identify an ionic compound as acidic, basic, or a neutral salt. You need to know the 6 common strong acids su... In this video we will look at the equation for H2SO4 + H2O and write the products. There are two reactions we need to consider since both of the hydrogens... A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion). For the SO3 2- Lewis structure the total number of valence electrons ... A step-by-step explanation of how to draw the NO3- Lewis Structure (Nitrate Ion). Get more chemistry help at www.Breslyn.org.For the NO3- Lewis structure,... A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure (Carbonate ion).For the CO3 2- structure use the periodic table to find the total nu... Trick to Find Conjugate Acid and Conjugate Base / Ionic Equilibrium Tricks A step-by-step explanation of how to draw the ClO3- Lewis Structure (Chlorate Ion). The ClO3- Lewis structure is a good structure to help you understand w... A quick explanation of the molecular geometry of ClO4 - (Perchlorate ion) including a description of the ClO4 - bond angles.Looking at the ClO4- Lewis struct... A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion).For the SO4 2- structure use the periodic table to find the total numb...
Copyright © 2024 hot.realmoneygametop.xyz